SILA are committed to compliance with the European REACH regulation—Registration, Evaluation and Authorisation of chemical substances.
What is REACH?
REACH stands for Registration, Evaluation and Authorization of Chemicals and is the name of the EU
Chemicals Regulation (1907/2006/EC) which entered into force on June 1, 2007 and which is intended to
centralize and simplify current chemicals legislation throughout Europe. REACH will end the current
international divide in the world of chemicals between new and existing substances.
The aim of REACH is to improve the protection of human health and the environment from the risks
associated with chemicals while enhancing the competitiveness of the chemicals industry within the EU.
REACH is based on the principle: “No data, no market”. For this, the substances currently on the EU market,
numbering around 30,000, will need to be evaluated systematically for their toxic and ecotoxic properties,
their applications studied along the entire product chain, including possible exposures, and appropriate
risk-reducing measures developed if necessary.
Affected by REACH are all manufacturers, importers and downstream users of substances and compounds
in quantities above 1 ton per year per Legal Entity.
Under certain conditions, products are also affected by REACH.
How will REACH work?
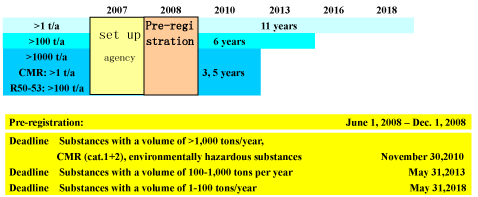
Pre registration
Pre-registration is a requirement for use of transition periods for the registration of phase-in substances
(existing substances, “EINECS”). For this, each manufacturer/importer must notify their substances with the
substance name, incl. CAS/EINECS no. (if available), address and volume band, to an agency in Helsinki
within six months of June 1, 2008. On the basis of this list, which will be published on the Internet one
month later without any company names being mentioned, the option will exist for companies to register a
substance jointly if they have an interest in doing so.
Registration
Each substance >1 ton per year must be registered if it is to be allowed to continue to be manufactured or
imported within the EU after June 1, 2008. For phase-in substances, transition periods apply (see Page2).
For registration, a technical dossier must be prepared with information on chemical industry + hazards, on
manufacture + use, on toxicological studies and test proposals. At volumes above 10 tons per year, a safety
report is also required. This documents the evaluation of substance safety for all specified applications over
the entire life of a substance. For this, it is absolutely essential for a communication system to be set up
along the entire product chain of a substance to enable all relevant data to be obtained on use/exposure.
Authorization
A key element of REACH is authorization, the gradual substitution of substances which are of particular
concern (CMR1+2, PBT and vPvB substances) with suitable alternative substances where this is
economically and technically feasible. Intermediate products (including monomers) and R&D substances are
exempt from authorization.
These substances will be included via a candidate list with an expiry date in Annex IVX of the Regulation. If
they are to continue to be used, a company-and use-specific application for authorization must then be submitted to the agency. For this, an analysis of alternatives and, if necessary, a socioeconomic analysis, is
required.
It can be assumed that the candidate list published on the Internet will be used as a basis for a “black listing”
of these substances on the market side.
On the part of Sila Chemicals, it is therefore recommended to start identifying possible substances requiring
authorization and to look for alternative substitute substances as of now.
What does the timetable look like?
What has Sila Chemicals done?
■ To introduce REACH, we have set up a team to specially implement the Regulation
■ Identified all substances manufactured and their corresponding volumes
■ Joined necessary associations worldwide to share knowledge and experience
What is Sila Chemicals doing next?
■ Pre-registration of all commercially available substances
■ Preparation of a customer questionnaire regarding additive usage etc.
■ If necessary development and marketing of potential replacement substances / recipes / formulations
■ Commence registration process within the necessary timeline
Your contracts at Sila Chemicals:
zhangtiehong@silachem.com
Mr. Zhang Tiehong Tel.+86(0)411-8376-9600,
Jack.kraclauer@silachem.com
Mr. Jack Kraclauer Tel.+86(0)411-8376-9000, |


